Where Does Photosynthesis Occur Which Primary Pigment Absorbs Red and Blue Light
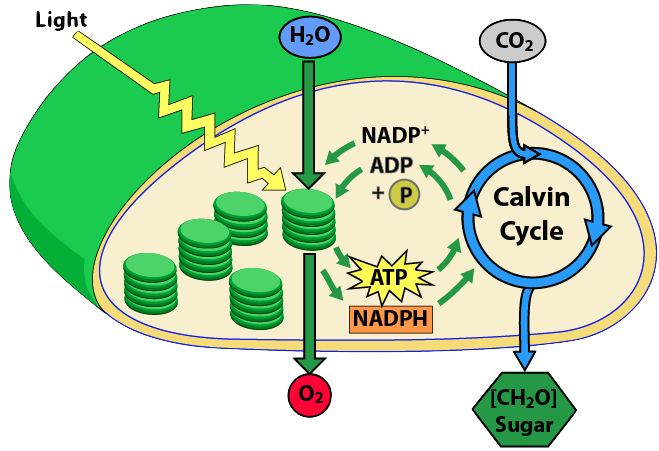 In the last module, we learned that photosynthesis occurs in two phases: the light reactions and the Calvin cycle. Here's a summary of what happens in each.
In the last module, we learned that photosynthesis occurs in two phases: the light reactions and the Calvin cycle. Here's a summary of what happens in each.
Now, let's delve into the details of the light reactions.
Energy is the ability to do work or cause change. In photosynthesis, it takes energy to change carbon dioxide (a low-energy exhaust product) into carbohydrate (a high-energy fuel). Photosynthesis harvests this energy during the light reactions, which can be thought of as a series of energy conversions.
Light is a form of electromagnetic energy. We commonly experience other forms of electromagnetic energy in our daily lives, including radio waves, microwaves, infrared light, ultraviolet light, and X-rays.
As a biology student, you only need to know a few things about electromagnetic energy.
The diagram above illustrates the connection between electromagnetic energy, wavelength, and frequency. In terms of photosynthesis, it also shows how visible light is just one portion of the electromagnetic spectrum. It's the portion that our primate visual system evolved to see, and which photosynthetic organisms evolved to exploit for converting electromagnetic energy into chemical energy.
Like all electromagnetic energy, light consists of photons that have a wavelength and a frequency (though only the wavelength is indicated in this image). Note that red light has a longer wavelength (700 nm) than violet light (400 nm). That means that red light has less energy than violet light does.
 Also, note that in our daily lives, we rarely experience light of only one wavelength. White light from a light bulb is a mixture of wavelengths, as is the light from the sun (which also includes many other wavelengths of electromagnetic energy, ranging from radio waves to X-rays). When we shine a beam of white light through a prism, however, we can see how it breaks up into its constituent colors. In nature, that happens whenever we see a rainbow.
Also, note that in our daily lives, we rarely experience light of only one wavelength. White light from a light bulb is a mixture of wavelengths, as is the light from the sun (which also includes many other wavelengths of electromagnetic energy, ranging from radio waves to X-rays). When we shine a beam of white light through a prism, however, we can see how it breaks up into its constituent colors. In nature, that happens whenever we see a rainbow.
3. Chlorophyll: the key photosynthetic pigment

When light (and all electromagnetic energy) interacts with matter, the energy can be reflected or absorbed. Substances called pigmentsabsorb certain light wavelengths and reflect others. So, what does it mean for something to be green, blue, or yellow? Because we're dealing with photosynthesis, which is carried out by green plants, let's focus on green. A green object is perceived as green because it has pigments that reflect green light (which bounces off the pigmented object into our eyes) and absorb other wavelengths of light. That means that the green leaves of plants are reflecting away green light, and absorbing other wavelengths, such as blue and red.
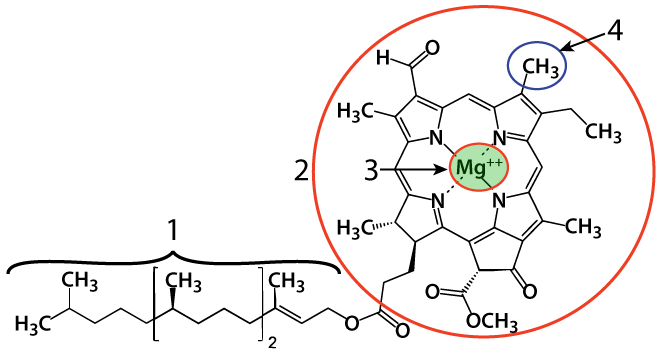
In chloroplasts, the primary photosynthetic pigment is chlorophyll. Chlorophyll molecules have a central magnesium atom (at "3"), essential for the process of converting light energy into electrical energy. The magnesium atom is embedded within a nitrogenous porphyrin ring ("2"). A long hydrocarbon chain anchors chlorophyll in the thylakoid membrane.
The methyl group (-CH3) shown at "4" is what makes this chlorophyll "chlorophyll a." Another chlorophyll, chlorophyll b, has the same structure, except for that at number "4" it would have a carbonyl group (a –C=O: carbon double bonded to an oxygen) instead of the methyl group. That chemical change has the effect of changing which wavelengths of light the chlorophyll absorbs, as we'll see below.
4. Absorption and Action Spectra
Chlorophyll's properties as a pigment can be quantified through its absorption spectrum. In a laboratory setting, you can take isolated chlorophyll, and, using a device like a spectrophotometer, you can measure which wavelengths of light chlorophyll best absorbs. Why is this important? Because the wavelengths of light that a pigment absorbs can be harvested to perform some work. In the case of photosynthesis, the work is creating an electrical current, which can be used to transform lower energy molecules into higher energy molecules.
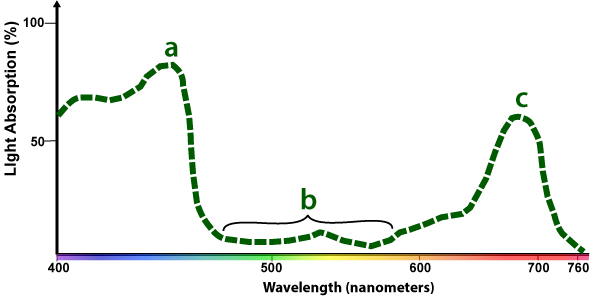
Here's the absorption spectrum of chlorophyll a. The X-axis shows the wavelength of light shining on the chlorophyll, and it ranges from short-wavelength violet light at 400 nm ("nm" = nanometer, or 1 billionth of a meter) on the far left to 760 nm red light on the far right. The Y-axis is the percentage of absorption of light energy.
The part of the line bracketed by "b" shows how in the green and yellow part of the spectrum chlorophyll a absorbs hardly any light. By contrast, the highest point of the line ("a") is where most light energy is absorbed (blue light). A second peak shows up in the red part of the spectrum at "c."
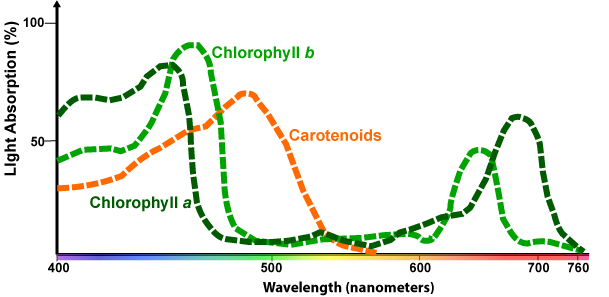
The graph below adds two additional photosynthetic pigments: Chlorophyll b and carotenoids. Carotenoids are accessory pigments: they help absorb light energy during photosynthesis, and they protect the photosynthetic pigments (in much the same way that the pigment melanin protects DNA in our skin cells from damage from ultraviolet radiation).

The absorption spectrum for a pigment shows how much light energy a pigment absorbs. By contrast, the action spectrum shows how much photosynthesis occurs at different wavelengths of light. In other words, an absorption spectrum is a property of a pigment. An action spectrum is a property of a photosynthesizing organism (or of isolated chloroplasts).
How could you measure how much photosynthesis occurs at different wavelengths of light? It's pretty straightforward, and there are many AP, college, or high school level lab activities where you do exactly that. One involves using disks from spinach leaves (cut out with a paper hole punch) and placed in water. As the disks photosynthesize, they produce bubbles of oxygen, which makes them float. You can measure how long it takes for the disks to float to the top of a cup of water, and that gives you an indication of how much photosynthesis is occurring.
The action spectrum for photosynthesis looks like what you see below.
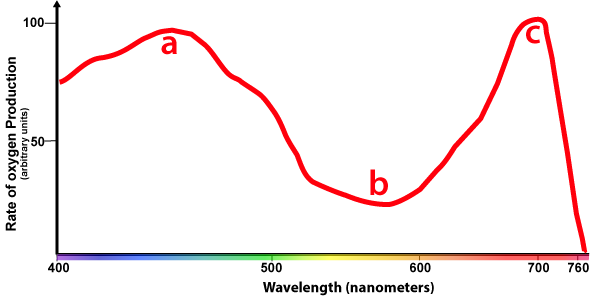
The X-axis of this graph is the same as the absorption spectra graphs above. The Y-axis now shows oxygen production. You can see that the highest amount of photosynthesis occurs at about 700 nm, in the red part of the spectrum (at "C"). Yellow light (at "b") generates the least amount of photosynthesis. Another peak is the blue part of the spectrum at "a."
hunterschanithems50.blogspot.com
Source: https://learn-biology.com/ap-biology/module-30-menu-photosynthesis/photosynthesis-3-light-and-pigments/
0 Response to "Where Does Photosynthesis Occur Which Primary Pigment Absorbs Red and Blue Light"
Post a Comment